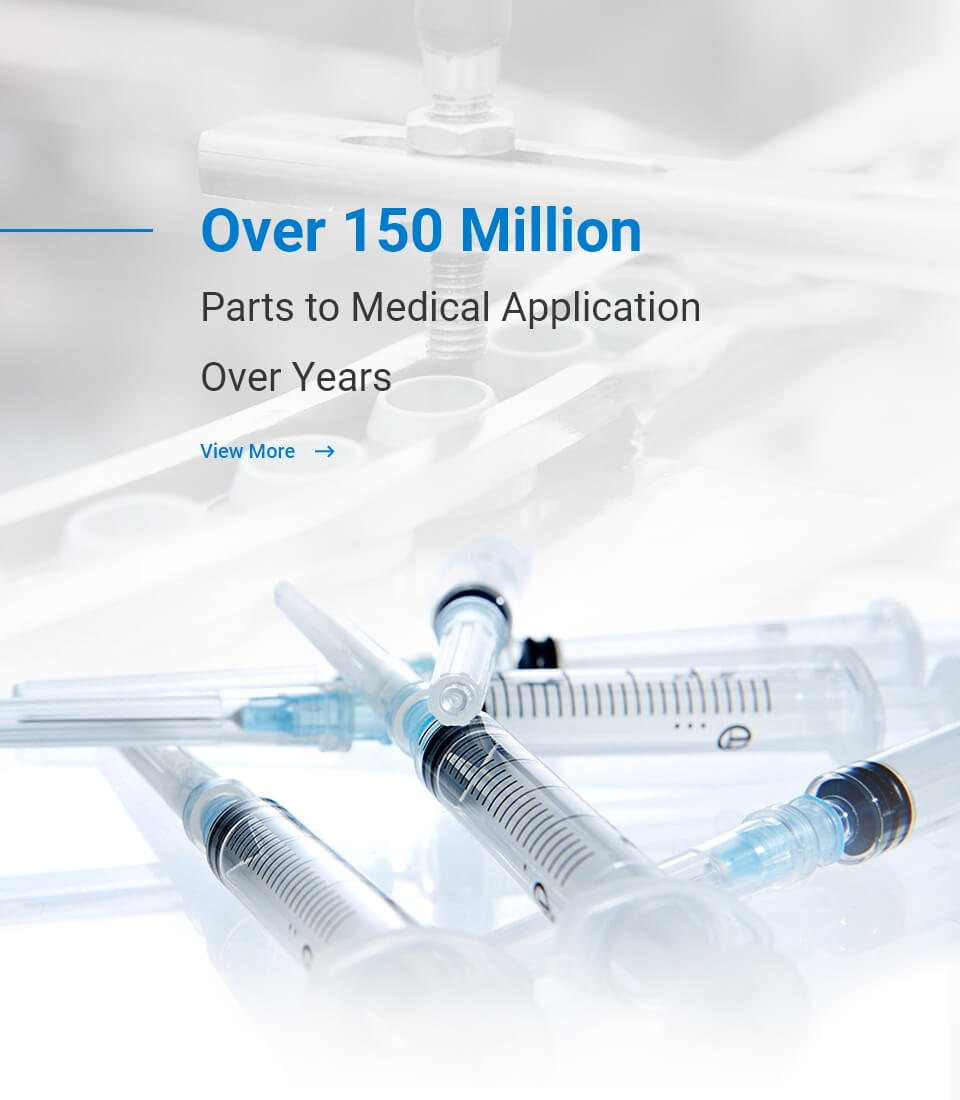
IMM provides controlled clean room environment and ISO13485 quality control system for
medical devices manufacturing.
Through the validation processes, IMM provides customer qualified and stable products to
the market. Please ink to our
website for our engineering capacity preview or feel free to contact us for more
possibilities.
I.V. Infusion
Enteral Feeding
Neuraxial Set
Irrigation tube Set
Cardio Tube
Insufflation tube